During the past 50 years remarkable gains have been achieved with the control of many infectious diseases. At the same time, new and previously unknown pathogens have emerged, and some, like HIV, have spread globally killing millions of individuals, disrupting societies, and reshaping the demographics of countries and regions. In addition, infectious diseases previously thought to be under control have re-emerged in many parts of the world. For purposes of this discussion, re-emerging infections are defined as those that have one or more of the following characteristics: increase in number of cases; expansion of current foci of infection or appearance in new geographic areas; appearance of infections in populations previously unaffected; and increase in severity of illness or mortality. This research paper explores the mechanisms through which infections re-emerge and the multiple factors in the world today that facilitate the reemergence of infections. Several specific infections are used as examples to illustrate key points. A discussion of the characteristics of infections that are most likely to reemerge in the future and a framework for preventing reemergence of infections, and thereby mitigating their consequences, concludes the paper.
Mechanisms For Re-Emergence
Multiple Factors Involved
Infectious diseases are dynamic; unless eradication of a microbe is achieved, which is rare, the interactions between microbes and humans undergo constant change and evolution. Microbes, with their rapid replication time, have the capacity to adapt to change much more rapidly than humans. At a fundamental level, infections that have been controlled re-emerge because: the microbe has changed, moved, or become more abundant; the host lacks or loses immunity (or capacity to respond to infection) or is not treated; or contacts between microbe and host increase. Although this may sound simple, multiple factors – biological, socioeconomic, demographic, and environmental – influence this dynamic relationship.
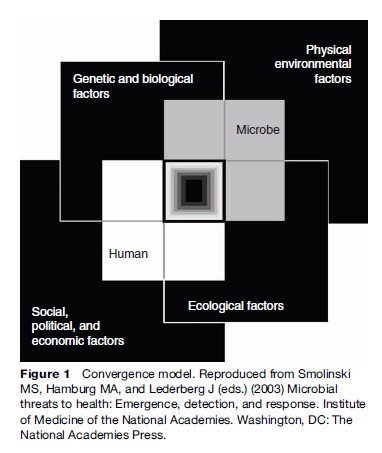
The convergence model (Figure 1) illustrates the broad context and interlocking domains of determinants in which infections emerge or re-emerge. Although the interactions between the human host and microbe are at the center of the process, other factors interact with each other and affect host, microbe, and their interactions. Disease emergence is often complex with multiple interacting factors involved. The model aptly depicts the central area of overlap as a black box, illustrating gaps in our understanding of many of the elements and how they interact.
For an infection to be defined as re-emerging, it must be recognized and characterized. It is important to acknowledge that many infections persist, reappear, and spread silently. Unless adequate clinical and laboratory facilities exist to accurately diagnose infections, they may go undetected or be categorized as a viral illness or flu. Microbes that cause infections that produce clinical signs and symptoms (such as fever and cough, fever and diarrhea, fever and muscle aches) similar to those of many common infections – such as influenza, tuberculosis (TB), salmonellosis, dengue fever, and malaria – may not be identified, especially in resource-poor settings where clinical laboratory support is absent or limited. Microbes that cause unusual clinical findings (e.g., vesicular skin eruption in monkeypox), high mortality (e.g., yellow fever), or produce large outbreaks (e.g., dengue fever and dengue hemorrhagic fever [DHF]) may be more likely to be identified.
Re-emerging infections are caused by all classes of pathogens (i.e., viruses, bacteria, fungi, helminths, protozoa) and involve pathogens with different modes of transmission (e.g., direct person-to-person transmission, airborne, vectorborne, food and waterborne) and different sources (e.g., another human, animal reservoir, soil and water). Typically multiple factors will have contributed to the re-emergence of a specific infection. These may vary by time or geographic region. Populations and regions vary in their vulnerability to the re-emergence of infections and capacity to intervene promptly, so to some extent re-emergence may be specific to time, place, and population. Because of the extensive linkages in the world today through trade and travel, re-emergence of an infection may have broader implications and pose greater risk to distant populations than it might have a few decades ago.
Changes In The Pathogen
The types of changes in a pathogen that can contribute to the re-emergence of an infection include development of resistance to antimicrobial agents that were previously effective, acquisition of new virulence factors or emergence of strains that are more virulent or transmissible, and emergence and spread of strains against which available vaccines are ineffective. Examples of each follow.
Resistance, Virulence, And Transmissibility
Resistance of a microorganism to an antimicrobial drug refers to the capacity of an organism to survive in the presence of that drug in therapeutic concentrations. Microbes can also be characterized by their resistance to killing by physicochemical conditions (e.g., heat, cold, acid, other) that may be relevant for survival in the environment, but these attributes are not discussed in this section. Virulence is a quantitative measure of pathogenicity of an organism or its likelihood of producing disease. Transmissibility refers to the ease of spread of a microbe from one host to another. These are three separate attributes that are not necessarily linked. For example, the H5N1 virus, the influenza strain currently circulating in avian populations, has shown resistance to the adamantane group of antiviral drugs. It is highly pathogenic in chickens and humans (and some feline species) causing high mortality in those species. In contrast, some infected ducks excrete the virus without showing symptoms of infection. As of early 2007, it is poorly transmissible from human to human, but highly transmissible in chicken populations. In general, many of the viruses that cause the common cold are highly transmissible from person to person but cause only mild illness.
Resistance
Increasing resistance of microbes to antimicrobials is occurring globally and involves microbes that cause millions of human deaths annually, including Staphylococcus aureus, Streptococcus pneumoniae (the cause of pneumococcal pneumonia and meningitis), Mycobacterium tuberculosis (the cause of TB), malaria parasites, influenza viruses, and human immunodeficiency syndrome viruses (HIV), among others.
Increasing resistance among some bacteria was initially localized primarily to tertiary care hospitals (e.g., methicillin-resistant S. aureus) but has now moved into the community and around the world. Increasing resistance is found among antimicrobials used to treat all types of infections, including viral, bacterial, fungal, and parasitic infections. In some instances, alternative agents are available for treating resistant infections, but alternative drugs may be more toxic, less effective, unavailable because of limited supplies, or expensive. High cost alone can mean many populations will be unable to have access to effective treatment, rendering infections operationally untreatable. In addition, resistance of arthropod vectors (e.g., mosquitoes) to pesticides has complicated the control of vectorborne infections, like malaria and dengue.
Bacteria can become resistant through mutations or by acquiring genetic material from related or unrelated bacterial species through horizontal exchange. Genetic changes in bacteria can occur in the absence of antimicrobials, but the presence of antimicrobials puts selective pressure on microbial populations. Resistant organisms may be able to flourish when an antimicrobial agent kills off other organisms that may compete for resources and may become the predominant population. Resistance traits are transferred to the progeny and potentially to unrelated strains of bacteria. The broad and indiscriminate use of antimicrobials has contributed to the rising resistance, but even appropriate use of antimicrobial agents puts pressure on microbial populations. In addition, certain clones or strains of resistant bacteria may become widely disseminated. With penicillin-resistant S. pneumoniae, for example, only 10 clones were shown to be responsible for 85% of invasive disease by this organism in the United States in 1998 (Corso et al., 1998). Travel and social networks may be important in the spread of resistant (or virulent) clones.
- aureus resistant to methicillin has expanded in geographic range, but until recently only five major clones were responsible for most of the worldwide problem. The microbe spread initially in defined groups with close contact – for example, children in child care facilities, athletes, intravenous drug users, prison inmates, and military recruits – then the organism disseminated into the general community. This may have been facilitated by special virulence factors of the organism that favored its survival (Furuga et al., 2006).
Resistance as a single factor cannot fully explain the re-emergence of TB or malaria, for example, but presence of resistance has contributed to increasing morbidity and mortality in some populations and makes control more complicated and costly.
Virulence
A previously uncommon but more virulent strain of Clostridium difficile, a cause of colitis that can be severe, has emerged to cause multiple outbreaks with substantial mortality in hospitals in North America and Europe. It produces 16–23 times more toxin than other strains of C. difficile, which may account for its increased virulence. It is also resistant to fluoroquinolones, and their wide use may have favored its emergence (McDonald et al., 2005).
An unusual example of a change in virulence has occurred in the attenuated live poliovirus vaccine. In rare instances (0.4–3 per million children vaccinated), the vaccine virus, which replicates in the gastrointestinal tract of the recipient, reverts to full neurovirulence. Not only can the vaccine-derived neurovirulent virus cause paralysis in the vaccine recipient, but vaccine-derived virus can also circulate in populations. Circulation of virulent vaccine-derived virus, in some instances with associated outbreaks of paralytic polio, has been documented in China, Egypt, Haiti, Madagascar, and the Philippines. Paralytic polio that has re-emerged in areas where circulation of wild poliovirus had ceased in some instances has been caused by vaccine-associated poliovirus (Kew et al., 2002). In other areas, wild poliovirus has been reintroduced from areas where polio persists in the human population. Geographic areas that appear to be at highest risk for circulation of vaccine-associated poliovirus are those with low vaccine coverage. Immunodeficient individuals (especially those with hypogammaglobulinemia) have been found to be long-term excreters of vaccine-derived poliovirus (in feces), one for at least 20 years (MacLennan et al., 2004). This remains a concern in making decisions about when it might be possible to stop using oral poliovirus and killed polio virus vaccines.
Strains, Serotypes, Serogroups, Subtypes
Microbes often exist as many different subtypes, serotypes, serogroups, or strains. This antigenic variation means persons who are immune (by natural infection or immunization) to one strain of an organism, may be susceptible to another. A previously uncommon serogroup of Neisseria meningitidis W135 (cause of meningococcal meningitis and sepsis) caused outbreaks among pilgrims to the Hajj in Saudi Arabia in 2000 and spread internationally. Because of previous outbreaks of meningococcal infections among the pilgrims (and subsequently their contacts in their countries of residence), Saudi Arabia started requiring all visitors to the Hajj to produce a certificate of vaccination against meningococcal disease upon entry into the country. Most visitors received a meningococcal vaccine active against serogroups A and C, the serogroups responsible for most epidemics of meningococcal disease in the past. It was in this context that meningococcal infections reemerged in the pilgrims in the spring of 2000, caused by serogroup W135. About 240 cases were reported in Saudi Arabia. In all, more than 400 cases in pilgrims and their contacts were identified in at least 14 different countries. Analysis of isolates from patients in France and the UK using multilocus sequence typing, DNA fingerprinting, and other techniques showed that they were indistinguishable from isolates from Saudi Arabia. Pilgrims who became infected could carry the W135 meningococcal clone and transmit infection, even if they did not develop symptoms. Of note, the vaccine used in the United States was a quadrivalent vaccine active against serogroups A, C, Y, and W135. Although the risk of disease was low in recipients of the quadrivalent vaccine, recipients could still become carriers of the outbreak strain as the vaccine does not prevent carriage (Dull et al., 2005). Saudi Arabia now requires pilgrims to the Hajj to have a vaccine active against W135. During February 2002, an epidemic caused by W135 began in Burkina Faso. By May more than 12 500 cases had been reported to the World Health Organization (WHO).
Dengue virus, a mosquito-transmitted flavivirus, is widespread in tropical and subtropical regions of the world and is expanding in geographic range and severity. Four different serotypes of dengue viruses cause disease in humans: DEN-1, DEN-2, DEN-3, and DEN-4. Infection with one dengue serotype is followed by only brief immunity to the other serotypes. Subsequently, if individuals are infected with a different dengue serotype, they are at increased risk (perhaps 100-fold greater) for severe disease, manifested as dengue hemorrhagic fever or dengue shock syndrome. In studies from Thailand, no cases of DHF were observed in patients with primary dengue infection, whereas 1.8–12.5% of those with a secondary infection developed DHF. The appearance of a new dengue serotype in a population that already has previously experienced high rates of infection with a different serotype may be followed by an outbreak of DHF. Infections that occurred a decade or more earlier may remain immunologically relevant and predisposed to severe illness. It is also becoming clear that dengue viruses vary in virulence, and introduction of a more virulent strain may be followed by particularly severe outbreaks.
Abundance
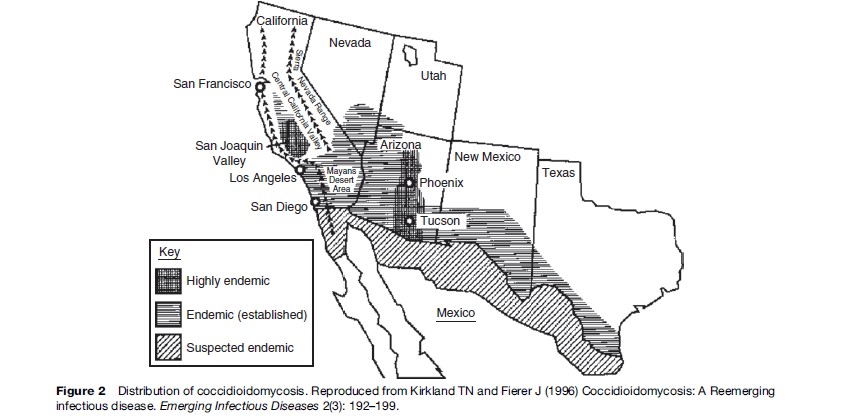
The presence (or absence) and abundance of many organisms is linked to the physicochemical environment, directly – for example, through temperature, rainfall, humidity, soil or water characteristics, pH, nutrients, and so on – or indirectly, for example, through the presence or abundance of arthropod vectors (e.g., mosquitoes), intermediate and reservoir hosts (such as rodents), and vegetation. Coccidioidomycosis, caused by a soil associated fungus, is found in arid and semiarid areas with alkaline soils in regions with hot summers and short, moist winters. Humans become infected by inhaling airborne arthroconidia. Growth of the fungi in soil is linked to temperature and rainfall. Outbreaks in the southwest United States have been correlated with the amount of rainfall over preceding months. The sequence of heavier than usual rainfall followed by prolonged drought in association with hot and dusty conditions favors dispersal of the fungus in a form that can be inhaled. But several other factors have also contributed to increasing cases of coccidioidomycosis in the U.S. southwest (Kirkland and Fierer, 1996). This area is one of the most rapidly growing parts of the United States; many new residents have moved from nonendemic areas, and thus lack immunity to the fungus. The area has become a popular place for retirement (Figure 2); persons more than 65 years of age have the highest incidence of infection of any age group. The HIV-infected population, another vulnerable group, has also grown in size. Land development, including construction, increases dust and potential exposures. Off-road vehicle use, which has become more common, also creates dust that can disperse the fungus. So although coccidioidomycosis is a ‘place’ disease that is influenced by geoclimatic conditions, the demographic shifts, human activities, and land development in the area have been important forces in the increase in cases. This disease would not have re-emerged if humans had not entered into and altered this environment.
An infection with a rodent reservoir host, such as hantavirus infection, can increase in response to geoclimatic conditions (Hjelle et al., 2000). The El Nino–Southern Oscillation (ENSO) has been associated with increased precipitation in the southwestern part of the United States. In this instance, the increased rainfall is associated with an expansion of the deer mouse population, presumably because of an expanded food supply for the mice, which are infected with the virus and excrete it in saliva, urine, and feces, typically without showing symptoms. Rodent populations can increase more than 10-fold in a year. More rodents mean more virus and more potential opportunities for human–rodent excreta contact and human infections.