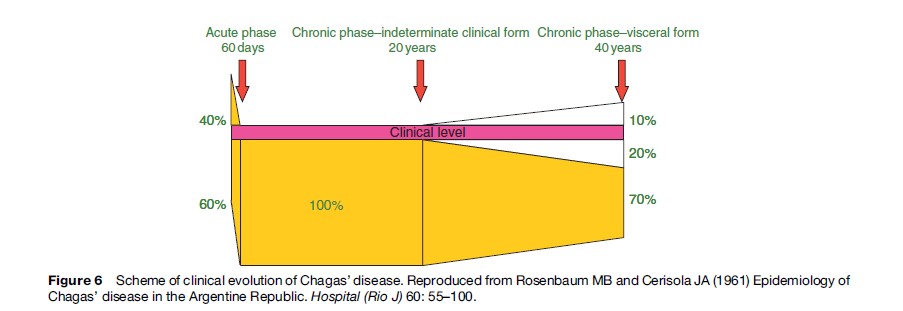
CD has two clinical phases: acute and chronic. The acute phase is undiagnosed in more than 90% of vectorborne cases. It can be symptomatic, oligosymptomatic, or asymptomatic. Some of the most frequent symptoms are febrile syndrome of long duration and lymphadenopathy. Some typical, though less frequent, symptoms are: Romana’s sign (unilateral bipalpebral edema), inoculation chagoma, hematogenous chagoma, and lipochagoma. Clinically overt, acute myocarditis or meningoencephalitis develops in approximately 1% of cases, mainly in children, and about one-tenth of cases are fatal (Lugones, 2002) (Figure 6).
Acute Phase
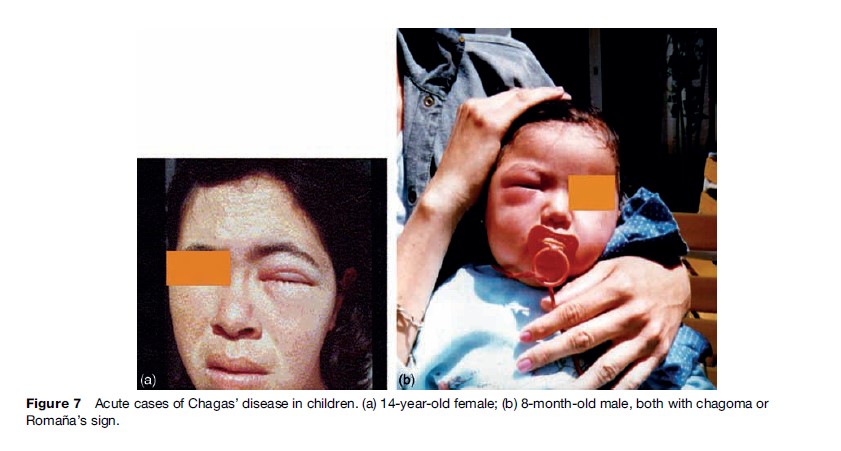
During the acute phase, diagnostic methods for T. cruzi infection focus on detecting the presence of the parasite or of specific T. cruzi antibodies. Some parasitological methods are concentration methods such as capillary microhematocrit, Strout, and other less sensitive methods, such as examination of a drop of fresh blood. These are useful during the acute phase of the infection, when parasitemia level is high (Figure 7).
Immunodiagnosis consists of serological reactions used to detect circulating antibodies to T. cruzi, primarily immunoglobulin G (IgG). IgG becomes detectable about 30 days after infection, and reaches maximum levels in the third month. Immunoglobulin M (IgM) is generated earlier, but is not always detectable. The absence of IgM antibodies against T. cruzi, epimastigote-derived antigens does not exclude infection. ELISA (enzyme-linked immunosorbent assay), IFA (indirect immunofluorescence), IHA (indirect hemoagglutination), gel particle agglutination, and – more recently – immunochromatography tests are used for IgG detection.
Following the acute phase, the great majority of infected people remain asymptomatic and with no clinical evidence of structural disease during the so-called indeterminate stage of CD. Most patients remain in this stage of CD for life, but antibodies to T. cruzi and low-grade parasitemia persist unless they are treated.
Approximately, 10–30% of infected patients after 2 or more decades of infection develop symptoms and signs of cardiac and digestive disease (Rosenbaum, 1961). To determine the presence of Chagas’ heart disease, an electrocardiogram (ECG) should be performed, and when abnormal, a chest radiograph or echocardiogram should be performed as well. Cardiac manifestation of CD includes abnormalities of the ventricular conduction system, ventricular arrhythmias, sinus node dysfunction, cardiac enlargement and dysfunction, heart failure, and left ventricular aneurysm. Of note, there is evidence from autopsy and biopsy studies indicating that subclinical, parasite-related myocarditis is present in 30% or more of subjects in this stage of CD who will never develop symptomatic disease.
Chronic Phase
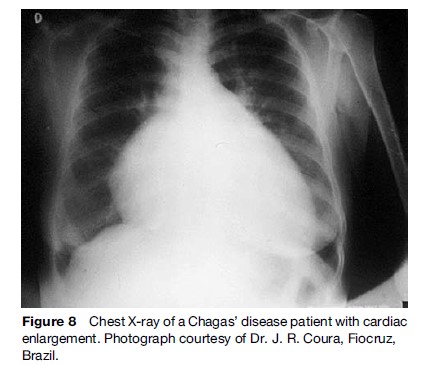
The major complications of chronic Chagas’ heart disease include signs of heart failure (usually with prominent systemic congestion and cardiac enlargement (Figure 8)), ventricular arrhythmias, and atrioventricular block. Chest pain – felt by 15–20% of patients – is usually atypical for myocardial ischemia, but in a subgroup of chagasic patients it may mimic acute coronary syndrome. However, epicardial coronary arteries are angiographically normal.
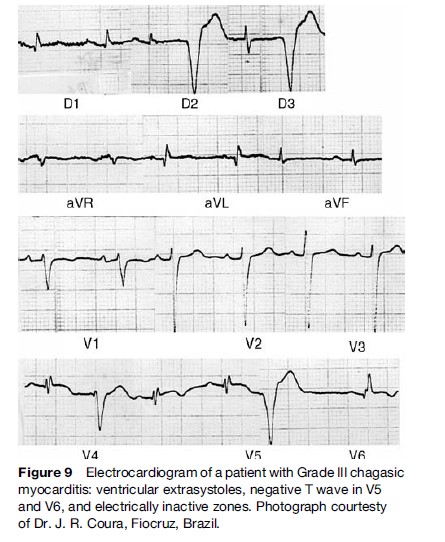
Typical ECG abnormalities (Figure 9) include right bundle branch with left anterior fascicular block and ventricular extrasystoles. Episodes of nonsustained ventricular tachycardia (VT) are present on 24-hour ambulatory ECG monitoring in approximately 40% of patients with wall motion abnormalities, and in 90% of patients with heart failure (Rassi et al., 1995). Sustained VT can be induced through programmed ventricular stimulation in a substantial proportion of patients. Not infrequently, complex ventricular rhythm disturbances coexist with bradyarrhythmias, as described by Chiale et al. (1982), and when associated with impaired left ventricular function, constitute a major risk factor for sudden cardiac death.
Striking segmental wall motion abnormalities in both ventricles occur early in the development of CD. The most characteristic lesion is the apical aneurysm, but it is the posterior basal dysynergy that best correlates with the occurrence of malignant ventricular arrhythmia. The aneurysms are also sources of emboli (Figure 10).
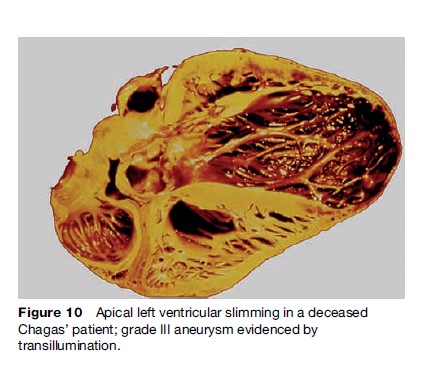
Mortality in patients with cardiac CD is primarily ascribed to sudden cardiac death, progressive heart failure, and thromboembolic events. Sudden cardiac death occurs in 55 to 65% of cases, progressive heart failure in 20 to 25%, and stroke in 10 to 15%. Sudden death is more frequent in young patients. The estimated total annual mortality caused by CD is 21 000 cases.
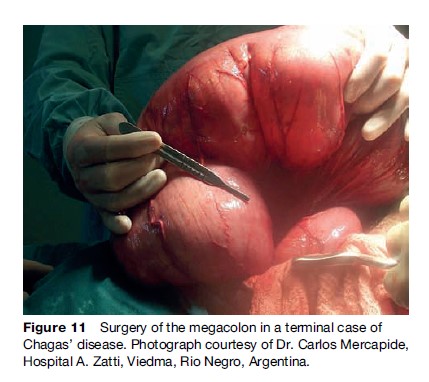
The gastrointestinal manifestations are a progressive enlargement of the esophagus or the colon, caused by chronic inflammation and destruction of parasympathetic neurons. The main gastrointestinal symptoms are dysphagia and severe constipation. Esophageal disease can appear as early as the acute phase of infection in children, while colonic disease evolves more slowly Figure 11.
Damage to the nervous system and striated muscle in the chronic stage, although not very clear in regard to its clinical significance, is expressed through motor cell loss or degeneration in various muscles, with infiltration and demyelination areas in the nerves. In the CNS, circumscribed or necrotizing inflammatory injuries can be found in the gray matter.
The diagnosis of T. cruzi infection during the chronic phase is best made by serological tests that detect circulating IgG antibody. There are useful amplification methods to establish the presence of parasites such as blood culture and xenodiagnosis, although the methods are significantly less sensitive during the chronic phase. The xenodiagnosis method used for many decades is now practically restricted to isolation work in the research area.
Assays based on PCR, which detect T. cruzi nucleic acids, have proved to be useful for verification of the parasite persistence in the host, and particularly useful for assessing the effectiveness of antiparasitic treatment.
Antiparasitic Treatment
So far it has been shown that treatment is effective for acute and congenital infections, reactivated infections in persons with HIV infection or receiving immunosuppressive therapy, and chronic infections of less than 15 years’ duration.
The effectiveness of treatment during the acute phase is easily demonstrated by the rapid drop of parasitemia and the disappearance of symptoms, signs, and circulating antibodies. Evaluation of the effectiveness of treatment is difficult in the chronic phase in which the disappearance of specific antibodies takes years, and this is the only accepted criterion of cure, as was established by a meeting of experts of the Pan American Health Organization (PAHO) (1999).
Several drugs have been tested for activity against T. cruzi, but only nifurtimox (1972) and benznidazole (1974), have demonstrated efficacy in human beings. These agents have been accepted by almost all Latin American countries’ Ministries of Health as treatment for T. cruzi infection. The objectives of treatment are to eliminate the parasite from infected persons, to diminish the probability of developing the disease, and to prevent T. cruzi transmission to others from infected persons (Sosa-Estani et al., 1998).
When trypanocidal treatment is used in patients in the late chronic phase, antibody titters may require 5 or more years to show a decline (Viotti et al., 1994). ‘‘Cure’’ rates in the late chronic phase, as determined by negative serology, are between 8 and 70%, and are higher for children than adults. Other assays that may be able to document cure sooner than conventional serology include serological tests employing recombinant antigen or purified, mucinlike glycoconjugate from cell-cultured trypomastigotes (De Andrade et al., 1996), xenodiagnosis, and PCR. Measurement of serum levels of the adhesion molecule p-selectin assays for interferon-gamma-producing T cells specific for a T. cruzi lysate group.
Several authors found that the results of treatment were more encouraging when ECG changes rather than serology were the outcome (Viotti et al., 1994; De Andrade et al., 1996). However, several other authors did not observe differences between treated and untreated patients. Based on the hypothesis that Chagas’ cardiomyopathy may indeed be triggered by persistent parasitic infection, it seems plausible that trypanocidal therapy may delay, reduce, or prevent the disease progress. This hypothesis must be tested by means of randomized clinical trials.
During treatment, patients must be under medical supervision to monitor for side effects, including cutaneous reactions, gastrointestinal intolerance, and, less frequently, central or peripheral neurotoxicity. Laboratory tests rarely show elevation of transaminases or leukopenia. Side effects are more frequently observed in adolescents and adults than in children and babies. In all cases, side effects disappear when the dose is diminished or the treatment is suspended.
It is necessary to search for better drugs than the ones available at this time to find a solution for the 16 million people infected with T. cruzi. Meanwhile, benznidazole and nifurtimox continue to be the only currently available drugs to treat persons with T. cruzi infection.
Control Of T. Cruzi Transmission
The main control strategies include surveillance and control of vectors, improvement of household construction, serological screening of blood and organ donors, and detection and treatment of congenitally infected infants.
Work contributed by Latin American researchers established the scientific basis for vector control and demonstrated its effectiveness in interrupting transmission. In 1948, Dias and Pellegrino described the successful use of the residual insecticide HCH (hexaclorocyclohexane) when applied to the walls of houses and peri-domestic structures infested with T. infestans in a rural area of Brazil. This vector has several attributes that facilitate its elimination: complete domiciliation in most endemic areas, and lack of sylvatic populations that could reinfest houses; low reproductive potential; and little capacity for active dispersion. In populations of Argentina (Segura, 2002), Brazil (D´ıas, 2002), and Venezuela having infestation levels higher than 15%, dwelling units have been treated with insecticides since the 1950s. More recently, regional control initiatives have extended vector control activities to other countries (see the section ‘Regional initiatives for control of transmission of T. cruzi ’).
A second approach to vector control is improvement of houses to eliminate the conditions necessary for colonization by triatomine bugs. These measures include plastering of walls and replacing thatch roofs to destroy potential hiding places for bugs, and providing the residents of rural areas with new, well-constructed houses. Such modifications or replacements were carried out in Venezuela in the 1970s and 1980s with excellent results, as well as in certain geographical areas in Bolivia and Paraguay with community participation. Reinfestation by Triatoma infestans after insecticide spraying has caused elimination efforts in all the regions to fail repeatedly (Cecere et al., 2006). Although house improvement and application of insecticide can be complementary, economic constraints have prevented more widespread implementation of measures to improve dwellings