The appearance of and spread of infection with HIV has had a profound effect on the burden from many other infectious diseases, most notably TB. The interaction between HIV/AIDS and TB is bidirectional, with each making the other worse. Infection with M. tuberculosis upregulates HIV replication, increases viral load, accelerates the decline of CD4 count, leads to more rapid progression of HIV, and increases the risk of death. The impact of HIV on TB is to increase the risk of primary infection, increase risk of rapid progression, increase likelihood of reactivation in those with latent infection, and increase the risk of reinfection. About one-third of humans are infected with M. tuberculosis, though infection is latent and asymptomatic in the majority. In most individuals the likelihood that TB infection will progress to active disease is only 5–10% over a lifetime. In persons co-infected with HIV and TB the risk of developing active TB may be as high as 10% per year. In areas where the incidence of TB was already high, the spread of HIV infection has led to an increase in incident cases of TB. Although the spread of HIV infection is not the only reason for re-emergence of TB in many areas, it is an important one – and one that has made control of TB much more difficult.
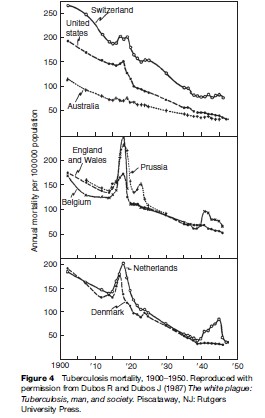
Data collected during the first half of the twentieth century on TB mortality showed notable increases in TB deaths during World War I and World War II, including in some countries that were not occupied (see Figure 4). This was an era before specific antituberculosis drugs were available. Increases in mortality were thought to relate to poor nutrition (especially limited protein supply), crowding in dark dwellings, and stress. Of note, Denmark was not occupied and did not take part in the armed conflict, yet TB mortality rose starting in 1915 (Figure 4). The rise in TB coincided with a drop in meat consumption, as meat was exported to England to support the war. Submarine attacks interrupted exportation from Denmark in 1917, making meat available for local consumption – and TB mortality fell (Figure 4).
Even today with good drugs available for the treatment of TB, social, political, and economic factors can influence treatment and outcome. TB has been a problem in disadvantaged populations, including inmates in prisons and homeless populations. Gustafson and colleagues (2001) described events in Guinea-Bissau, West Africa, where armed conflict, starting in 1998, limited access to TB treatment. This was followed by an increase in the mortality rate ratio (MR) (3.21) among those being treated for TB and was most pronounced among those who were HIV infected (MR 8.19).
Contacts Between Host And Microbe
Many factors can lead to an increase in contact between host and microbe. The outbreaks of infectious diseases, such as shigellosis, cholera, and measles in refugee camps and in other displaced populations, indicate how rapidly infections can spread when basic supports, such as clean water, adequate sanitary facilities, and immunization, are unavailable (Goma Epidemiology Group, 1995).
Hepatitis C
Although a serologic test to identify persons infected with hepatitis C virus (HCV) was not developed until 1989, the infection had been described based on its clinical and epidemiologic features, and by the absence of antibodies to other common infections, including hepatitis A and hepatitis B. Before the wide use of tests to screen donated blood, hepatitis C was the most common blood-borne infection following blood transfusions. Although hepatitis C is found worldwide, prevalence varies widely. Seroprevalence in Egypt has been reported to be in the range of 20% in blood donors, one of the highest prevalence rates anywhere in the world. Prevalence varies by age and geographic region, but is higher in Egypt than in neighboring countries. The HCV subtypes in Egypt are mostly the same, suggesting epidemic spread throughout the country. Children have a low prevalence of infection and people living in desert areas have the lowest prevalence. Investigators have tried to find plausible explanations for these observations.
Schistosomiasis, a parasitic infection that requires snails as an intermediate host, is widespread in the Nile Delta and Nile Valley. After it was discovered in 1918 that injections with an antimony salt could kill schistosomal flukes residing in humans, use of parenteral antischistosomal therapy (PAT) became widespread. Because the drug could be administered only by injection and multiple injections (12–16) were required, mass treatment campaigns were organized to deliver the drug. Sterilization procedures of reusable injection equipment were likely to have been omitted in some instances or were inadequate. When effective oral therapies for schistosomiasis became available in the 1970s and 1980s, they replaced PAT. Investigators who have analyzed the prevalence of HCV antibodies by age and geographic region have found a significant association between exposure index for PAT and HCV prevalence rates. This suggests that the mass campaigns to treat schistosomiasis may have effectively spread HCV to a significant portion of the Egyptian population (Frank et al., 2000). Although initial infection with hepatitis C may be mild or asymptomatic, infection becomes persistent in 50–80% of those infected. The burden of disease related to infection, progression to cirrhosis or development of hepatocellular carcinoma, comes years or decades after infection was acquired. Unsafe injection equipment can be a source of infection in any country, but the prevalence of infection is less than 2–3% in most countries.
Although safer, more effective treatment for schistosomiasis is now available, schistosomiasis has increased in some communities in Egypt, Senegal, Cote d’Ivoire, and elsewhere following the building of dams (N’Goran et al., 1997). Dams can have potential economic and health benefits, but have also been associated with increases in vectorborne infections (see the following section).
Malaria
Malaria continues to kill a million or more, mostly young children in Africa, each year, and saps the strength and productivity of individuals in endemic areas by causing fatigue, episodic fevers, and anemia. Although in the overall area of the globe where malaria is endemic the disease has decreased by half in the last century, demographic changes have resulted in an increase of 2 billion people living in areas with malaria risk (Hay et al., 2004). The intensity of transmission has also increased in some regions. Some reasons for increase in malaria include insecticide resistance of the mosquito vector and breakdown in control programs, increase in resistance of the malaria parasite to the inexpensive, older antimalarial drugs (e.g., chloroquine), and population movements (both movement of nonimmune populations into malarious areas and also migration of malaria-infected persons into regions infested with competent malaria vectors and climatic conditions compatible with malaria transmission). Co-infection with malaria and HIV also appears to have adverse consequences, though not of the magnitude noted with HIV and TB. HIV infection is associated with an increased risk of parasitemia and clinical malaria in adults and increased risk of treatment failure. HIV-infected pregnant women have more frequent and higher-density malaria parasitemia than do uninfected women; infants born to women infected with both malaria and HIV have a threeto eightfold higher risk of postnatal death than infants born to mothers with either infection alone. Malaria in HIV-infected persons also leads to increased viral load, which returns to baseline with prompt treatment of malaria.
Because malaria is transmitted by a mosquito vector – environmental factors, including temperature, humidity, rainfall, and land use – can also influence abundance and location of mosquito vectors. A study in northwestern Ethiopia linked an increase in malaria transmission to intensified maize cultivation. Maize pollen, eaten by mosquito larvae, can influence the insect’s size and development. In many villages, maize was planted in fields less than 2 meters from houses. The predominant variety planted, chosen for its high yield, matured late, with pollen dispersal extending into the peak period of mosquito larval development (Kebede et al., 2005).
In the Peruvian Amazon in South America malaria prevalence increased sharply in the 1990s at a time of population migration and growth as well as extensive deforestation. Researchers gathered data from 56 different study sites that varied by type of vegetation and human population density. They captured mosquitoes that landed on human volunteers in the evening hours to assess whether deforestation was associated with increased biting rate of Anopheles darlingi, the primary vector of falciparum malaria in that region. They found that human-biting rates were consistently higher in deforested areas, with the A. darlingi biting rate more than 278 times higher in deforested areas than in areas that were predominantly forested (Vittor et al., 2006).
Dams built to provide irrigation for crops can also provide water for breeding of mosquito vectors. In a longitudinal study of communities living near dams and control communities at similar altitude but beyond the flight range of mosquitoes that might breed in standing water created by dams, researchers found that malaria was significantly more common in communities near the dams (14.0 episodes in 1000 child months vs. 1.9 in control villages) (Ghebreyesus et al., 1999).
Introduction of a mosquito species that is a more efficient vector for malaria transmission can also lead to an increase in transmission. An example of this occurred when Anopheles gambiae was introduced into Brazil, probably by way of boats that made mail runs between Dakar, Senegal and Natal, Brazil. A. gambiae larvae were first identified in March 1930 and subsequently spread along the coastal region and 200 miles inland into the Jaguaribe River valley. Although malaria was endemic in this area, the local mosquitoes were not efficient vectors, so the human burden from malaria was low. The establishment of the highly efficient vector, A. gambiae, was followed in the late 1930s by explosive malaria outbreaks with more than 100 000 cases and about 20 000 deaths in this population with low levels of immunity. A massive campaign to eradicate A. gambiae was successful, but the event illustrated the key role of the type of vector present in malaria transmission.
Movement Through Trade
Movement of microbes through travel and trade continues to lead to outbreaks in unexpected places and populations. In many instances, transmission cannot be sustained and the infection dies out. Infections that are transmitted from person to person can potentially be carried to any country, as has been observed with HIV. Influenza, though never absent, re-emerges every year in temperate climates during the colder months to cause epidemics. The severity of the epidemics depends on the extent of change in the virus (genetic drift or shift), and to some extent on use of vaccine in the population, and closeness of match between the vaccine virus and circulating virus. Traveling, infected humans introduce the virus into new geographic areas, where it can spread rapidly because it is highly transmissible. The H5N1 avian influenza that has caused massive die-offs in poultry and other birds, since it was first identified in 1997 in Hong Kong, can also be carried by migratory birds to new countries and continents (Rappole et al., 2000). Legal and illegal movement of poultry and trade involving pet birds and fighting cocks are other routes of spread.
Dengue And Mosquito Vectors
Humans are the main reservoir for dengue virus (primates are not significant in its epidemiology in most areas). They spread it into new geographic areas by traveling while infected (virus in bloodstream) and being bitten by mosquitoes competent to permit the replication of the dengue virus to levels in which it can be transmitted via mosquito saliva when it bites a nonimmune host many days later (typically a week or two for the extrinsic incubation period). Dengue has increased in geographic reach and severity and is now present in most tropical and subtropical areas of the world. Several factors are contributing to its re-emergence, with the increased travel of humans being an essential but insufficient reason. Much of global travel is by plane, allowing travelers to reach anywhere in the world within the incubation period of dengue fever, typically 4 –7 days (full range is 3–14 days). The most important dengue vector is Aedes aegypti, and it now infests most tropical and subtropical areas. It thrives in urban areas and is able to breed in discarded plastic cups, flowerpots, and used tires and enters homes and prefers a human host. The greatest volume of population growth today is occurring in urban areas in low latitude regions, and in areas infested with a competent vector and linked to the global community via travel and trade. More and more urban areas in tropical regions have reached the population size (estimated to be 150 000 to 1 million) needed to sustain the ongoing circulation of dengue virus.
Of note, presence of a competent vector and a climate that is warm enough to permit the virus to become infective in mosquitoes is not sufficient to lead to outbreaks of dengue fever. The mosquito vector must have access to the human population. Presence of screens and air conditioning can limit transmission as a study by Reiter and colleagues (2003) showed, which looked at two urban areas (Laredo, Texas and Nuevo Laredo in Mexico) with similar climate and separated only by the Rio Grande River. A. aegypti was present in both areas. In a serosurvey, residents in Mexico were significantly more likely to have antibodies indicating recent or remote dengue infection than were residents across the border in Texas. Absence of air conditioning was significantly associated with dengue antibodies. Poor housing is also associated with increased exposure to mosquitoes, and the absence of piped water can also increase risk of exposure to mosquitoes because water storage vessels kept in the household can serve as good breeding sites for mosquitoes.
Mosquitoes are also moved around the world on ships, airplanes, and other vehicles. In some instances, the species can become established in a new geographic area. Aedes albopictus, the Asian tiger mosquito, was introduced into the United States in 1985 in used tires imported from Asia and within 12 years had spread to at least 25 states. It has also recently been introduced into many areas in Latin America. It was the primary vector responsible for the outbreak of dengue fever that occurred in Hawaii in 2001–02 and is also competent to transmit West Nile and other viruses that can cause severe disease in humans.
Monkeypox
Monkeypox, a viral infection that resembles smallpox, was first recognized in captive primates in 1958 and first identified in humans in 1970 in the Democratic Republic of the Congo. Infections that occurred earlier were probably diagnosed as smallpox because of similar clinical findings. Only after smallpox had been eradicated from this area and surveillance for smallpox-like infections was instituted was this virus identified. Unlike smallpox, whose only host is the human, monkeypox is a zoonosis, though human-to-human spread can occur. It is not as easily transmitted as smallpox among humans, and transmission is usually not sustained beyond 2–3 generations. Although primates, including monkeys, can be infected, other animals, perhaps squirrels and other rodents, are thought to be the reservoir hosts for this virus. The virus is found in tropical rain forest areas of west and central Africa. Because vaccination with vaccinia virus gives partial protection against monkeypox, some investigators have speculated that monkeypox might increase after the eradication of smallpox and cessation of the vaccination.
No one expected monkeypox to appear in North America in 2003, though in retrospect public health officials should not have been surprised. Exotic animals, such as the Gambian giant rat, imported from Ghana had been housed by distributors with prairie dogs sold as pets in the United States. At least 37 human monkeypox infections were documented in pet dealers and owners and veterinarians. Humans became sick after contact with sick prairie dogs. No humans died; in African outbreaks mortality has ranged from 4 –22%, perhaps because of greater virulence of the virus subtype. There have been no additional cases since 2003 or any evidence that the virus has become established in prairie dogs or other animal populations in the United States.