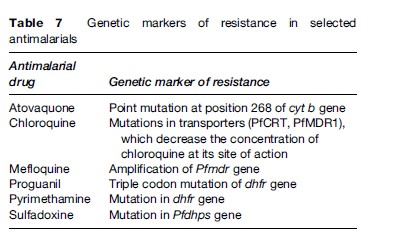
In most areas of the world where malaria is transmitted, it is caused by drug-resistant parasites (Table 6). Antimalarial resistance has been described in all species of Plasmodium infecting humans, except P. ovale. P. falciparum resistance to most antimalarials, with the exception of artemisinin derivatives, has been documented. However, in only a few of these drugs can genetic markers of resistance be identified (Table 7). Chloroquine resistance in P. falciparum is widespread, with efficacy confined to P. falciparum-endemic areas north of the Panama Canal in Central America and the Caribbean. Mefloquine and multidrug resistance in P. falciparum is, for the most part, confined to the Thai–Burmese and Thai–Cambodian borders, along with parts of Vietnam and eastern Burma. P. falciparum resistance to quinine monotherapy has been reported in Southeast Asia, but is of little consequence when quinine is used as a component of combination therapy.
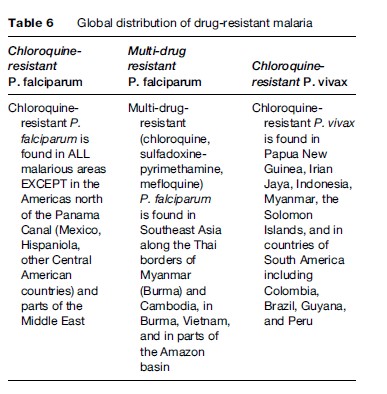
Chloroquine resistance in P. vivax is mainly found in Papua New Guinea and Papua (Irian Jaya), along with other countries of Oceania such as Indonesia, East Timor, and the Solomon Islands. There have also been isolated reports of chloroquine-resistant P. vivax from the Amazonian regions of Peru, Brazil, and Guyana. Chloroquine remains an effective antimalarial for most P. vivax acquired in Southeast Asia (other than in Thailand, Korea, and Myanmar), subcontinental India, the Middle East, and Latin America. Only recently has chloroquine resistance in P. malariae from Indonesia been described (Maguire et al., 2002). To date, P. ovale remains fully sensitive to the existing antimalarial pharmacologic armamentarium.
Treatment is considered to have failed if fever and parasitemia fail to resolve or recur within 14–28 days of treatment initiation. These failures may arise due to drug-resistant organisms, malabsorption of the drug (vomiting or diarrhea), or poor adherence. A full course of retreatment with an alternate regimen is indicated in these cases. If fever and parasitemia recur >2 weeks post-treatment, this likely reflects recrudescence, or reinfection in an endemic zone.
Prevention
Prevention In Travelers
Malaria is a preventable disease in travelers. A general approach to the prevention of malaria in travelers involves an assessment of individual risk, a discussion of mosquito bite avoidance measures, and the prescription of chemoprophylactic agents where appropriate.
Assessment Of Individual Risk
Many factors contribute to an individual’s risk of acquiring malaria when traveling and include, but are not limited to:
- Geographic destination;
- Type of travel (urban vs. rural);
- Type of accommodations (tents vs. screened rooms);
- Itinerary during travel (trekking, altitude, river or jungle exposure);
- Season of travel (wet vs. dry);
- Duration of travel (short-term vs. long-term);
- Likelihood of compliance with preventive measures.
All travelers to malarious areas should receive specific pretravel counseling from a qualified professional who can provide an educated assessment of the individual traveler’s risk of acquiring malaria abroad, and who can address the following two pillars of malaria prevention.
Mosquito Bite Avoidance
Use of personal protective measures and behaviors to reduce the likelihood of being bitten by a female anopheline mosquito is key to the prevention of malaria. These measures and behaviors include, but are not limited to:
- Avoidance of outdoor activity after dusk (Anopheles mosquitos bite from dusk from dawn);
- Use of long-sleeved shirts and long pants when exposure is likely;
- Use of N,N-diethyl-3-methylbenzamide (DEET)based mosquite repellants;
- Use of permethrin or other insecticide-impregnated bed net.
Aerosol, pump, and gel-based formulations containing 20–50% DEET can be used safely and efficaciously in adults and children over 2 months of age. Picardin is another commonly used repellant that is safe and effective in both adults and children, and tends to cause less skin irritation than DEET. Insecticide-impregnated bed nets can be used safely by both pregnant women and children. Permethrin is also available in liquid or spray formulations for impregnation of clothing.
Chemoprophylactic Agents
Following a detailed assessment of individual risk of malaria and counseling around personal protective measures for bite avoidance, the pretravel encounter can be directed toward a needs assessment for chemoprophylaxis. In order to prescribe an appropriate agent, the travel medicine practitioner will want to determine:
- If the traveler will be exposed to malaria;
- Which species of Plasmodium predominates in his or her region of travel;
- If there is likely to be drug-resistant P. falciparum at his or her destination;
- Whether or not the traveler is likely to adhere to the prescribed regimen;
- If there are any contraindications to the prescribed regimen such as pregnancy or likelihood of pregnancy;
- Whether or not the traveler will have ready access to medical attention during travel.
A detailed past medical history is important to gather in order to determine if there are contraindications to a particular class of antimalarials.
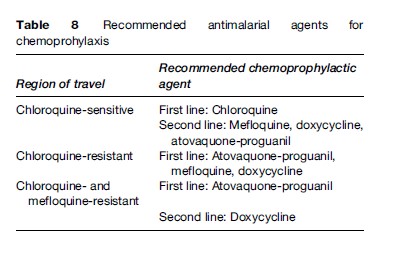
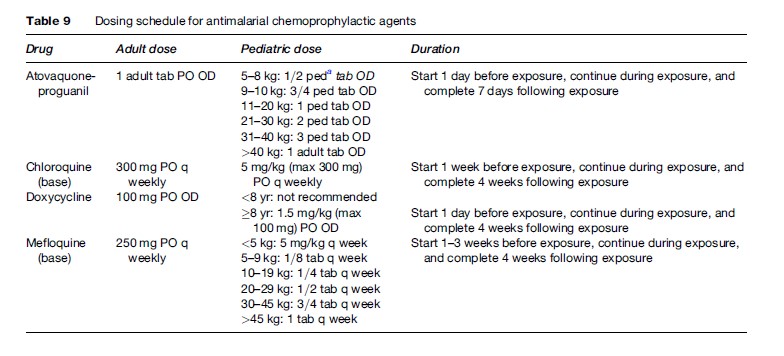
Tables 8 and 9 summarize the antimalarials that are currently recommended for chemoprophylaxis in travelers to malarious regions. It is important to emphasize to the traveler that all chemoprophylactic regimens must be started prior to arrival in the risk area, taken throughout travel in the risk area, and continued for one to four weeks post-travel, depending on the regimen. Drugs such as atovaquone-proguanil that work by preventing the preerythrocytic development of the parasite (causal prophylaxis) need only be taken one week post-travel. Conversely, drugs such as mefloquine, chloroquine, and doxycycline that work by suppressing blood stage infection need to be taken for a full four weeks post-travel.
In travelers who will not have timely access to medical care, standby therapy (self-treatment) can be life-saving. Self-treatment may also be an appropriate option for those in whom the chemoprophylactic regimen is suboptimal due to underlying medical condition. Options for standby therapy include atovaquone-proguanil, chloroquine (in a chloroquine-sensitive region), or quinine plus doxycycline. Dosing is as outlined in Table 4. It should be emphasized to travelers that self-treatment in no way obviates the need for timely medical attention, nor should it be perceived as an alternative to prophylaxis. In addition, travelers should be discouraged from purchasing self-treatment regimens overseas or from switching their prophylactic regimen while abroad.
Prevention of malaria in the pregnant traveler presents a challenge due to widespread chloroquine resistance in P. falciparum, and the lack of chemoprophylactic agents that can be used safely in all trimesters. Chloroquine can be safely used during all trimesters of pregnancy, and is appropriate for prophylaxis against chloroquine-sensitive P. falciparum. The decision to travel to an area with high transmission of chloroquine-resistant P. falciparum while pregnant should not be taken lightly. P. falciparum malaria can have grave consequences for mother, fetus, and neonate. If transmission is thought to be high and likelihood of exposure will be great, and travel cannot be deferred, then mefloquine prophylaxis is a reasonable option. While there is mounting evidence that atovaquoneproguanil is likely to be safe in pregnancy, insufficient data are available to recommend its use as a prophylactic agent in pregnancy. Doxycycline and primaquine are contraindicated in pregnancy.
Prevention In Residents Of Endemic Areas
Due to the prohibitive costs and infrastructural requirements of mass prophylaxis campaigns, insecticide-treated bed nets and targeted chemoprophylaxis remain the mainstays of malaria prevention in endemic areas. Children less than 5 years of age and pregnant women are candidates for intermittent preventive therapy (IPT) or continuous chemoprophylaxis. IPT consists of twice or thrice pre-emptive therapy during the course of pregnancy (or infancy) with an agent such as chloroquine or chloroquine-proguanil. This strategy in pregnancy reduces the risk of severe malarial anemia, low birth weight, and severe disease in pregnant women, though these benefits are largely seen in primigravidae. Insecticidetreated bed nets have been shown to significantly reduce the burden of childhood mortality secondary to malaria, and to reduce the incidence of anemia and malaria in pregnancy.
Malaria Vaccine
Malaria vaccine initiatives have been ongoing for over 30 years now; however, only recently have candidate malaria vaccines been tested in humans in clinical trials. To date, investigational vaccines have been designed to target specific stages of the parasite (notably P. falciparum) life cycle, including the preerythrocytic/liver stages, asexual erythrocytic stages, sexual blood stages, and mosquito stages. The complexity of the parasite life cycle has hampered the development of successful candidate vaccines, as immunity to one life cycle stage (e.g., liver stage) confers no protection to other stages (e.g., blood stages). Natural immunity to P. falciparum is highly strain-specific and ephemeral, and requires multiple episodes of infection (boosting) to maintain both humoral and cell-mediated immunity. That P. falciparum expresses approximately 5300 antigens further hinders vaccine development, as it is currently unknown which of these antigens are key players in the genesis of immunity.
One preerythrocytic stage vaccine, RTS,S/AS02, has shown promise in early clinical trials. This vaccine was designed to target the malarial circumsporozoite protein, and the vaccine antigen is comprised of a fusion protein (RTS) expressed in yeast, which binds to hepatitis B surface antigen (S) to form RTS,S. When mixed with an adjuvant, AS02, and given intramuscularly to volunteer vaccinees, RTS,S induces a high-titer antibody response to both circumsporozoite protein and hepatitis B surface antigen. In a randomized controlled trial in the Gambia, adults given three doses of RTS,S/AS02 were protected from developing natural P. falciparum malaria (Bojang et al., 2001). Vaccine efficacy was 71% in the first nine weeks of the surveillance period, and 34% during the entire 15-week surveillance period (Bojang et al., 2001). No protection was afforded by the vaccine in the final six weeks of surveillance, reiterating that immunity is very short-lived. In a follow-up study, it was shown that the protection conferred by RTS,S/AS02 was not strainspecific (Alloueche et al., 2003). Additional studies are ongoing in Mozambique. While there are many other candidate vaccines entering the early stages of clinical evaluation in humans, RTS,S/AS02 is the first to demonstrate efficacy in natural P. falciparum infection. These results are very promising, and a commercially available vaccine is on the horizon.
Bibliography:
- Alloueche A, Milligan P, Conway DJ, et al. (2003) Protective efficacy of the RTS,S/AS02 Plasmodium falciparum malaria vaccine is not strain specific. American Journal of Tropical Medicine and Hygiene 68: 97–101.
- Bojang KA, Milligan P, Pinder M, et al. (2001) Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: A randomized trial. Lancet 358: 1927–1934.
- Maguire JD, Sumawinata IW, Masbar S, et al. (2002) Chloroquineresistant Plasmodium malariae in south Sumatra, Indonesia. Lancet 360: 58–60.
- World Health Organization (2000) Severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 94(supplement 1): 1–90.
- World Health Organization (2006) Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization.
- Baird JK (2005) Effectiveness of antimalarial drugs. New England Journal of Medicine 352: 1565–1577.
- Franco-Paredes C and Santos-Preciado JI (2006) Problem pathogens: Prevention of malaria in travelers. Lancet Infectious Diseases 6: 139–149.
- Greenwood BM, Bojang K, Whitty CJM, and Targett GA (2005) Malaria. Lancet 365: 1487–1498.
- Hewitt K, Stekete R, Mwapas V, et al. (2006) Interactions between HIV and malaria in non-pregnant adults: Evidence and implications. AIDS 20: 1993–2004.
- Leder K, Black J, O’Brien D, et al. (2004) Malaria in travelers: A review of the GeoSentinel surveillance network. Clinical Infectious Diseases 39: 1104–1112.