The first human case ascribed to infection with T.gondii was a child with hydrocephalus reported by Janku in 1923 ( Janku, 1923). Sabin reported the first case of encephalitis due to T. gondii (Sabin, 1941), and encephalitis due to T. gondii in immunocompromised patients was first reported from patients with Hodgkin’s disease during immunosuppressive treatment (Flament-Durand et al., 1967). During the 1940s there was improved understanding of the cause of maternal infection for congenital toxoplasmosis in newborns, and in 1953, Feldman reported a series of 103 children, 99% of whom had eye lesions, 63% had intracranial calcifications and 56% had psychomotor retardation (Feldman, 1953). This initiated interest in congenital infection among scientists in Europe (Couvreur, 1955).
In Gothenburg, Sweden, 50% of mothers had had previous infection with T. gondii and 2 out of 23 260 children had clinical toxoplasmosis during a 1948–51 study period (Holmdahl and Holmdahl, 1955). A study from Austria reported frequent symptoms in children with congenital toxoplasmosis (Eichenwald, 1957). A French study concluded that treatment prevented transmission from mother to child and reduced the clinical symptoms in children (Couvreur and Desmonts, 1962). A later study from France found that the seroprevalence in pregnant women in Paris was 85%, and there was a high risk of toxoplasma infection in seronegatives (Desmonts et al., 1965; Desmonts and Couvreur, 1974).
Following these studies, systematic prenatal screening programs were introduced in France and Austria in 1975 (Aspo¨ck and Pollak, 1992; Thulliez, 1992). The use of toxoplasma-specific IgM antibodies for neonatal diagnosis was proposed in 1968 (Remington et al., 1968), and systematic neonatal screening was piloted in New York (Kimball et al., 1971). The first neonatal screening program based on detection of IgM antibodies at birth was initiated in New England (U.S.) in 1988 (Guerina et al., 1994).
Prevalence Of T. Gondii Infection
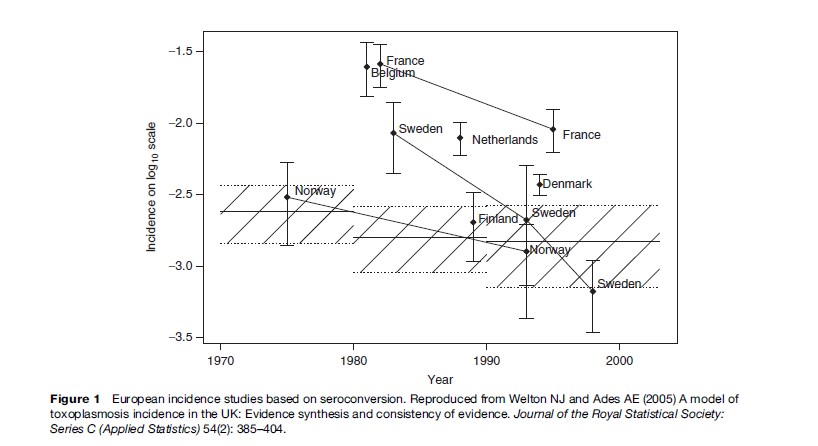
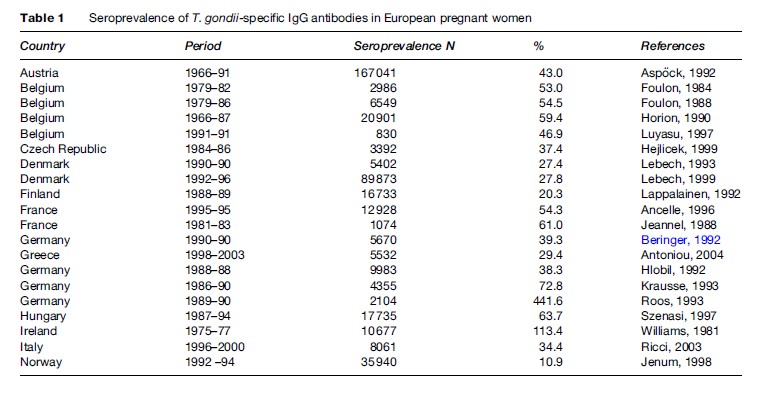
The prevalence of toxoplasma infection in Europe has recently been reviewed by Be´nard and Salmi (2006a), and data from different countries are shown in Table 1. The prevalence of infection has been decreasing in Europe over the past 3 to 4 decades (Horion et al., 1990; Forsgren et al., 1991; Krausse et al., 1993; Logar et al., 1995; Breugelmans et al., 2004; Welton and Aedes, 2005) (Figure 1). In the United States, data are collected regularly through the National Health and Nutrition Examination Study (NHANES) study. NHANES III 1999–2000 found a T. gondii seroprevalence of 15.8% in the age group 12–49 years. T. gondii seroprevalence was higher among non-Hispanic black persons than among non-Hispanic white persons (age-adjusted prevalence 19.2% vs. 12.1%). No statistically significant differences were found between T. gondii antibody prevalence in NHANES 1999–2004 and NHANES III (1988–94) ( Jones et al., 2003; McQuillan et al., 2004).
In South America, a study from Brazil found that seroprevalence was high in people living in poor socioeconomic conditions probably due to waterborne transmission (BahiaOliveira et al., 2003). Another study found a seroprevalence of 73% in slaughterhouse workers and suggested that fresh meat is a significant source of infection in Brazil (Dias et al., 2005). A study of children from Guatemala found that infection with T. gondii often took place before the age of 5 years at which age 43% were seropositive ( Jones et al., 2005). These data show that in countries where waterborne infections are prevalent, infection occurs at an early age.
A study from Korea found an immunoglobulin G (IgG) prevalence in pregnant women of 0.8% (Song et al., 2005), and a recent study of HIV-positive patients from Taiwan found a seroprevalence of 10.2% (Hung et al., 2005). A recent study from India found a seroprevalence of toxoplasma-specific IgG antibodies of 45% (Singh and Pandit, 2004), and a study of HIV-infected patients from Japan found an overall seroprevalence of 44.8%. The majority of these patients were in the age of 25 to 34 years (Nissapatorn et al., 2004). A study of 327 adult cat owners in Thailand found a seroprevalence of 6.4% (Sukthana et al., 2003), and a study from Malaysia found a high seroprevalence in Malays of 55.7% and people belonging to the Indian ethnic group of 55.3%, but low in ethnic Chinese, at 19.4% (Nissapatorn et al., 2003).
A study from Sao Tome´, West Africa, found a prevalence of 21.5% in children below 5 years of age (Fan et al., 2005) and a study from Sudan found a seroprevalence in pregnant women from Khartoum of 34.1% (Elnahas et al., 2003). Of 1828 HIV-positive patients from Bobo-Dioulasso, Burkina Faso, 25.4% had positive T. gondii serology (Millogo et al., 2000).
T. Gondii Genotypes And Clinical Disease
- gondii can be divided into three main genotypes (Sibley and Boothroyd, 1992; Grigg et al., 2001; Khan et al., 2005). It has been proposed that the different genotypes may be partly responsible for the different pathogenicity observed in the infection. In mice one T. gondii parasite of genotype I is lethal to mice, whereas the lethal dose of genotype II and III is about a thousand parasites (Boothroyd and Grigg, 2002). One study has reported an unusual abundance of type I and recombinant strains in patients with retinochorioditis (Grigg et al., 2001), and a recent study from Brazil of eyes with T. gondii lesions from necropsies found only type I and III strains and no type II strains (Vallochi et al., 2005). Recent work, however, suggests a more complicated picture in Brazil with both pathogenic and nonpathogenic isolates belonging to genotype I (Ferreira et al., 2006). A study of 86 pregnant women from France found predominantly genotype II (Ajzenberg et al., 2002), which confirms previous studies that also found primarily genotype II (Howe et al., 1997).
Recently, methods have been developed that allow T. gondii in patients to be at least partially typed using genotype specific markers (Kong et al., 2003), and this will be a valuable tool for assessing the geographical distribution of genotypes as well as the importance of genotype for pathogenicity. The phylogenic development over time of the different genotypes suggests that the ‘atypical’ or ‘exotic’ genotypes may be the ancestral types and genotype I, II, and III are more recent developments from the ancestral parasite (Su et al., 2003). A series of 16 cases with symptomatic T. gondii infection were reported from French Guyana, infected with T. gondii genotypes that did not belong to genotypes I, II, or III (Carme et al., 2002). The presently available data suggest the genotype II dominates in Europe, genotypes I and III dominate in South America, and all three genotypes can be found in the United States and Canada (Ajzenberg et al., 2004; Peyron et al., 2006).
Risk Factors For Infection With T. Gondii
Epidemiological surveys that examine the risk factors in infected and noninfected persons remain the most valid way of assessing the relative importance of different sources of T. gondii infection in humans (Leroy and Hadjichristodoulou, 2006). No biological test can distinguish infection from oocysts transmitted by felines from infection with tissue cysts in infected meats (Dubey, 2000; Hill and Dubey, 2002). Soil contact through gardening allows contact with infective oocysts deposited by cats. Oocysts take 1 to 5 days to become infective, but they can remain infective in soil and probably water for up to 1 year depending on ambient temperature and humidity (Frenkel et al., 1975).
A prospective, case-control study from Norway in 1992–94 found that eating raw or undercooked meat and meat products, poor kitchen hygiene, cleaning the cat litter box, and eating unwashed, raw vegetables or fruits were associated with a higher risk of T. gondii infection (Kapperud et al., 1996).
From 1991 to 1994 a prospective risk factor study in pregnant women infected during pregnancy and controls was performed in Italy. Eating cured pork or raw meat at least once a month was associated with a threefold higher risk of T. gondii infection (odds ratio [OR]: 3.1; 95% confidence interval [CI]: 1.6–6.0) (Buffolano et al., 1996). A case-control study from France found the following risk factors: poor hand hygiene (OR: 9.9; 95% CI: 0.8–125), consumption of undercooked beef (OR: 5.5; 95% CI: 1.1–27), having a pet cat (OR: 4.5; 95% CI: 1.0–19.9), frequent consumption of raw vegetables outside the home (OR: 3.1; 95% CI: 1.2–7.7) and consumption of undercooked lamb (OR: 3.1; 95% CI: 0.85–14) (Baril et al., 1999).
A European, multicenter, case-control study in Belgium, Denmark, Italy, Norway, and Switzerland included 252 cases and 708 controls (Cook et al., 2000). The study showed that contact with raw or undercooked beef, lamb, or other sources of meat, as well as with soil, were independent risk factors for T. gondii seroconversion during pregnancy. In addition, travel outside of Europe, the United States, and Canada was a risk factor for seroconversion. The population attributable fraction showed that 30 to 63% of seroconversions were due to consumption of undercooked or cured meat products and 6 to 17% were a result of soil contact, but ownership of a cat was not a risk factor (Cook et al., 2000). Information about how to avoid toxoplasmosis in pregnancy could be a cost-effective approach to preventing congenital toxoplasmosis (Conyn-van Spaedonck and van Knapen, 1992; Lopez and Dietz, 2000). Based on the knowledge of these identified risk factors for primary toxoplasmosis, pregnant women should be appropriately advised by their obstetricians and primary-care providers on how to lower the risk of congenital toxoplasmosis by avoiding risk factor exposure.
Transmission through surface water has been found to be important in Brazil, and this is probably an important source of transmission in poor socioeconomic societies in the tropics and subtropics (Bahia-Oliveiera et al., 2003).
Recommendations to prevent congenital toxoplasma infection in pregnant women are shown in Table 2 based on the EUROTOXO review of risk factors (Leroy and Hadjichristodoulou, 2006).
