Prenatal treatment consists of spiramycin and sulfonamides combined with pyrimethamine (Charpiat et al., 2006a,b,c). The effectiveness of spiramycin is doubtful (Peyron et al., 2000; Charpiat et al., 2006d). Prenatal treatment has no effect on maternal–fetal transmission of T.gondii or on clinical manifestations in infants infected with congenital toxoplasmosis (Gilbert et al., 2001; Gras et al., 2001; European Multicentre Study on Congenital Toxoplasmosis, 2003; Gras et al., 2005). These findings have been confirmed by a recent individual patient data meta-analaysis of cohort studies from across the world (Thie´baut et al., 2006c).
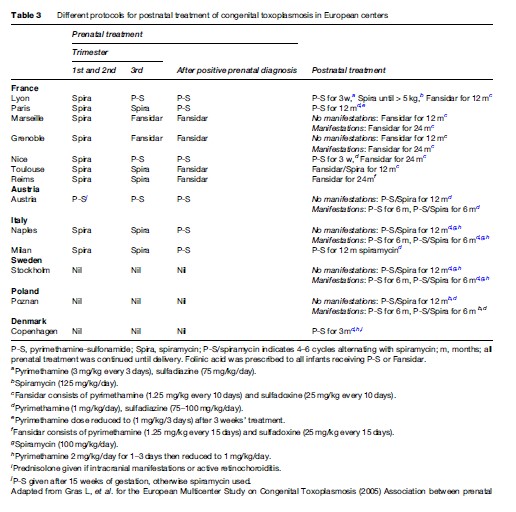
Table 3 shows postnatal treatment regimens used in different European centers. Postnatal treatment of congenital toxoplasmosis has recently been reviewed (Petersen and Schmidt, 2003). There are no randomized, controlled trials of treatment effect of either preor postnatal treatment.
New Drugs
The most promising new drug for the treatment of T. gondii is atovaquone, and studies in mice suggest that it may be partially effective against the tissue cyst (Huskinson-Mark et al., 1991). Azithromycin has also been found to have a partial effect on T. gondii tissue cysts (Derouin et al., 1992; Charpiat et al., 2006e).
Artemisinins have been tested in mice models and one study demonstrated that these drugs reduced brain cyst load (Sarciron, 2000). A study in the hamster model of T. gondii eye infections found no effect of atovaquone on the eye lesions but a 90% reduction in brain cyst numbers (Gormley et al., 1998). Other studies of atovaquone have also reported significant increased survival and reduction in brain cyst burden (Araujo et al., 1998; Alves and Vitor, 2005).
Prevention Of T. Gondii Infection
Infection with T. gondii is in theory preventable by interrupting the route of transmission of tissue cysts through meat and meat products and preventing infective (sporulated) oocysts in the environment from reaching humans. In practice, there are few data that show that health education significantly reduces rates of infection (Gollub et al., 2006).
In some places where prenatal screening has been implemented, a decline of congenital toxoplasmosis has been observed, as reported recently in Belgium, for instance (Breugelmans et al., 2004). The proportion of the decline specifically attributable to the program is unknown because no unscreened group of women exists for comparison, and there has been an overall decline in rates of seropositivity throughout Europe. Although established criteria for screening programs require evidence of effectiveness, no such evidence is available to support prenatal or neonatal screening.
Conclusion
The burden of T. gondii infection has been decreasing in Europe over the past 40 years, coinciding with the increased industrialization in farming.
The evidence for any effect of pre and neonatal screening programs rests on theoretical considerations and for programs started more than 30 years ago on data 40–50 years ago. With the declining risk in Europe and a lack of evidence of a treatment effect on maternal–fetal transmission, clinical disease in newborns, and a lack of evidence for a long-term reduction in eye disease in treated infants, there is a need for proper trials documenting the effectiveness of screening programs.
The small number of patients in each center, and the different approaches to diagnosis (screening or no screening) and treatment makes collaborative studies based on a common protocol mandatory to provide data that make rational decisions possible regarding diagnosis, treatment, and follow-up.
New methods of food production, especially organic meat where the animals are in contact with the environment, will increase the risk of T. gondii infection, which eventually will increase the risk of human infection.
Bibliography:
- Ahlfors K, Borjeson M, Huldt G, and Forsberg E (1989) Incidence of toxoplasmosis in pregnant women in the city of Malmo, Sweden. Scandinavian Journal of Infectious Diseases 21: 315–321.
- Ajzenberg D, Cogne´ N, Paris L, et al. (2000) Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. Journal of Infectious Diseases 186: 684–689.
- Ajzenberg D, Banuls AL, Su C, et al. (2004) Genetic diversity, clonality and sexuality in Toxoplasma gondii. International Journal for Parasitology 34: 1185–1196.